
tentukan senyawa yang bertindak sebagai oksidator Dan reduktor Dari reaksi Zn+CuSO4 menjadi - Brainly.co.id

How to Balance Zn + CuSO4 = Cu + ZnSO4 | Zinc plus Copper (II) Sulfate - YouTube

Zn (s) + CuSO4 (aq) => ZnSO4 (aq) + Cu (s) termasuk reduksi atau oksidasi. menggunakan caranya - Brainly.co.id

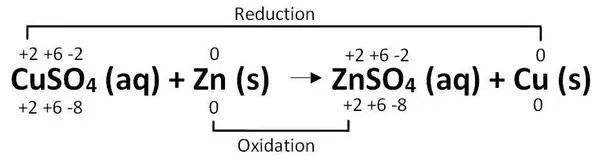
Zn(s) + CuSO4(aq) ZnSO4(aq) + Cu(s) - ppt download

Zn(s) + CuSO4(aq)→ ZnSO4 (aq) + Cu(s) - ppt download

What Type Of Reaction Is Zn+cuso4=znso4+cu

What Type Of Chemical Reaction Is Cuso4+zn=znso4+cu

How to Balance Zn + CuSO4 = Cu + ZnSO4 | Zinc plus Copper (II) Sulfate - YouTube

Type of Reaction for Zn + CuSO4 = ZnSO4 + Cu - YouTube

Zn(s) + CuSO4(aq)→ ZnSO4 (aq) + Cu(s) - ppt download

Tentukan zat yang bertindak sebagai oksidator dan reduktor reaksi: zn + cuso4 ➡ znso4 + cu
What Kind Of Reaction Is Zn+cuso4

Zn(s) + CuSO4(aq)→ ZnSO4 (aq) + Cu(s) - ppt download

Which of the following reaction is possible? Explain giving suitable example.1) Zn(s) + CuSO4(aq) - Brainly.in

during this time. 4. Zn + CuSO4 ZnSO4 + Cu Identify the oxidising and reducing agents. Give reason.
Apa persamaan seimbang untuk ZnSO4+Cu? - Quora

Net Ionic Equation for Zn + CuSO4 | Zinc + Copper (II) Sulfate - YouTube

Solved What is the standard cell potential for the reaction | Chegg.com

Zn+CuSO_(4)rarrZnSO_(4)+Cu, यह किस प्रकार की अभिक्रिया का उदाहरण है?
What Type Of Reaction Is Cuso4 + Zn

PERCOBAAN VIII REAKSI REDOKS (Kimia Dasar II) - Blog Najih

REAKSI REDUKSI OKSIDASI DAN ELEKTROKIMIA Dra M SETYORINI

Solution: Which statement is true for thi… | Chemistry

Solved What type of reaction is: CuSO4(aq) + Zn(s) → Cu(s) + | Chegg.com

Zn + CuSO4 = Cu + ZnSO4 | Balanced Chemical Equation

Cuso4 Aq ~ 35+ images cuso4 aq zn s cu s znso4 aq reaction, how to write the net ionic equation for ki cuso4 k2so4, a the student dissolves the entire impure

Type of Reaction for Zn + CuSO4 = ZnSO4 + Cu - YouTube

1.Tentukan senyawa yang mengalami okisadi dari persamaan redoks berikut Zn + CuSO4 > ZnSO4 + Cu2.Tentukan senyawa yang mengalami

a) Effect of Cu/ZnSO4, Cu/Zn-Mnt, and Cu/Zn-Thz on the intestinal… | Download Scientific Diagram

Calculate equilibrium constant for the following reaction : Zn+CuSO_(4) rarr ZnSO_(4) +Cu E_(Zn//Zn^(2+))^(@)=0.765” volt, “E_(Cu^(2+)//Cu)^(@)=0.347 volt

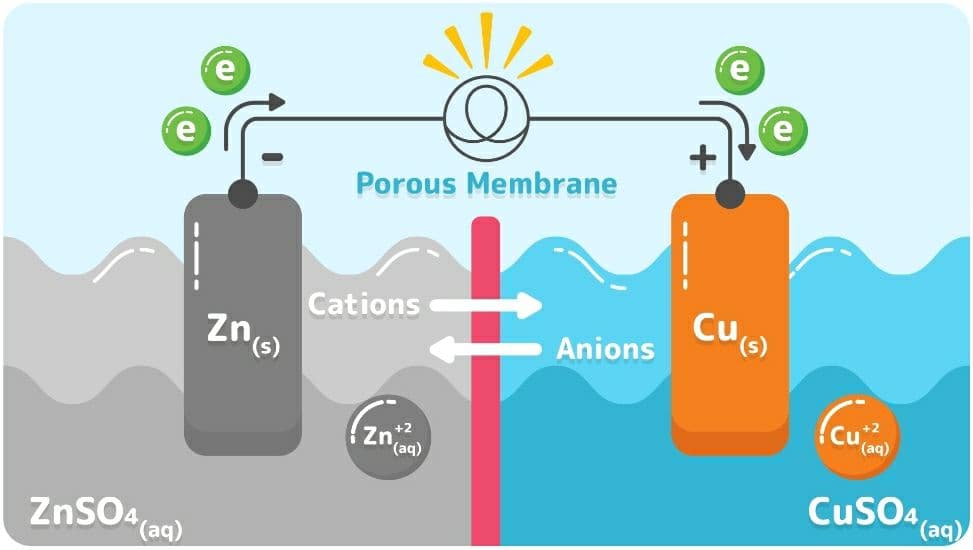
KIM-ASYIK: Sel Volta

Electrochemical processes - online presentation

Sel elektrokimia

CuSO4 (aq) + Zn (S) → Cu (S) + ZnSO4 (aq) is an example of - Chemistry Q&A

Zn(s) + CuSO4(aq) ZnSO4(aq) + Cu(s) - ppt download

Done 1423160_1557024700 (Chemistr 1. Consider the following reactions: Zn + CuSO4(aq) — > ZnSO4(aq) + Cu

PPT - Answers PowerPoint Presentation, free download - ID:6703346

What Type Of Reaction Is Zn + Cuso4

Reaksi redoks

Pin on Balance

Tentukan Reduktor, Oksidator, hasil oksidasi dan hasil reduksi dari suatu reaksi redoks beikut. Zn - Brainly.co.id

Zinc reacts with CuSO4 according to the equation Zn + CuSO4→ZnSO4 + Cu If excess of zinc is - Chemistry - Solutions - 12598611 | Meritnation.com
Solved Question 5 When the equation below is balanced, the | Chegg.com

How to Balance Zn+CuSO4=Cu+ZnSO4|Chemical equations Zn+CuSO4=Cu+ZnSO4|Zn+ CuSO4=Cu+ZnSO4 Balance - YouTube

Answered: What is the oxidizing agent in the… | bartleby
Penentuan Kalor Reaksi ZN | PDF

Zn Cuso4 ~ 35+ images photograph of commercial zn powder and cuso4 aqueous, ppt, cuso4 zn
Mengapa seng menggantikan tembaga dari tembaga sulfat? - Quora

Zinc reacts with CuSO_(4) according to the equation Zn+CuSO_(4)rarrZnSO_(4)+ Cu . If excees of zinc is added to 100.0 ml of 0.05 M CuSO_(4) the amount of copper formed in moles will be

Zat yang bertindak sebagai oksidator pada reaksi zn + cuso4 > znso4 + cu adalah? ada yang tau sekalian cara nya, buat besok ni.

Zinc reacts with with CuSO4 according to equation Zn+CuSO4=ZnSO4 +Cu if excess of zinc is added of zinc is added - Chemistry - Some Basic Concepts of Chemistry - 13524689 | Meritnation.com

a) Item 6skBb(Q1/Q2), (b) Item 7skBs(Q1/Q2) | Download Scientific Diagram

Gambarkan rumus kimia dari reaksi redoks - Brainly.co.id

Sel elektrokimia

In the chemical reaction, Zn (s) + CuSO4 (aq) ⇌ Z…
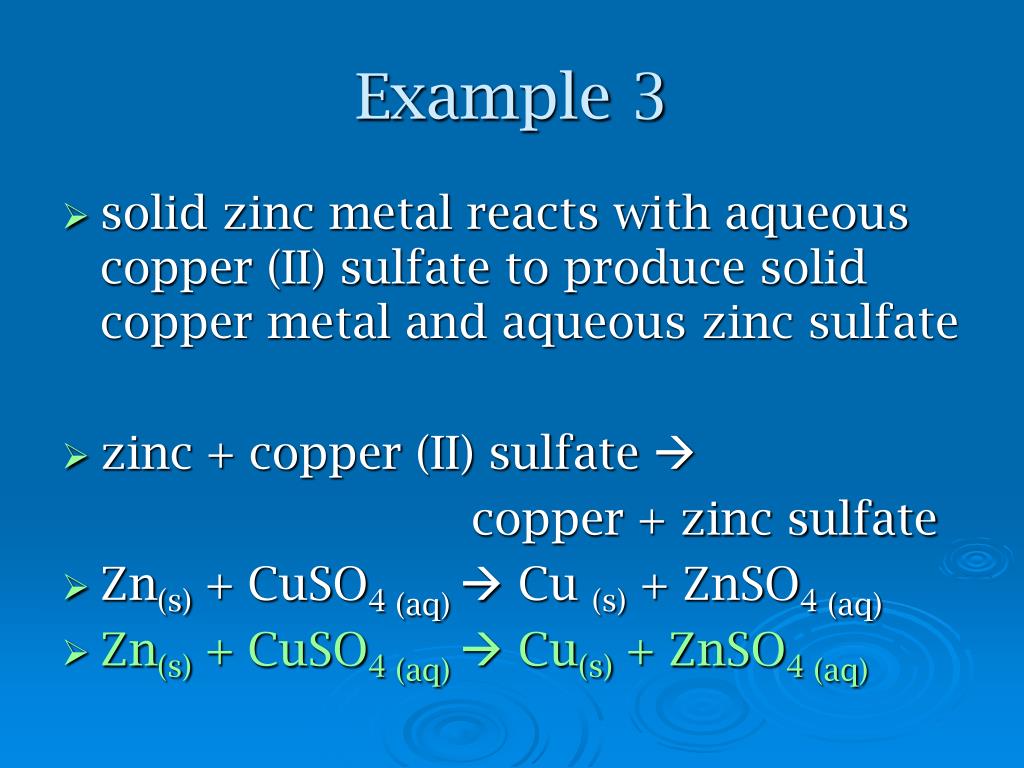
PPT - What is the difference between a chemical reaction and physical change? PowerPoint Presentation - ID:5813241

Copper to Copper lab - Money: Zn+CuSO4—>ZnSO4+Cu

Solved Question 7 Balance the equation: Zn + CuSO4 → ZnSO4 + | Chegg.com
Studi Awal Pembuatan Serbuk Tembaga Berstruktur Amorf

- Zinc reacts with CuSO. according to the equation Zn i Cu ? | Scholr™

ZnSo4 CuSo4 Zn Cu
In the electrochemical cell : Zn|ZnSO4(O.01 M)||CuSO4( 1.0 M)lCu, the emf of this Daniell cell is E1. - Sarthaks eConnect | Largest Online Education Community

Copper to Copper: Zn(s) + CuSO4(aq) –> Cu(s) + ZnSO4(aq)

What is the percent yield for a reaction i… | Clutch Prep

Answered: What is the oxidizing agent in the… | bartleby

PERCOBAAN SEL ELEKTROLISIS

Zinc reacts with CuSO_(4) according to the equation Zn+CuSO_(4)rarrZnSO_(4)+ Cu . If excees of zinc is added to 100.0 ml of 0.05 M CuSO_(4) the amount of copper formed in moles will be
Mengapa larutan Cuso4 menjadi tidak berwarna jika ditambahkan seng? - Quora

Solved 5. Which is the reducing agent in the following redox | Chegg.com
CuSO4(aq) + Zn(s)→ ZnSO4(aq) + Cu(s) is an example of decomposition reaction. It is true or false?

Pin on Geometry Of Molecules

tentukan bilok,reaksi reduksi,reaksi oksidasi,dari…

- Which is the reducing agent in the following redox reaction? CuSO4 + Zn - >… - HomeworkLib

cuso4+zn=ZnSO4+Cu расставить коофеценты методом электроного баланса и указать окислители и - Школьные Знания.com

PPT - 9. Oxidation-Reduction PowerPoint Presentation, free download - ID:1003963

SOLVED: When the equation below is balanced, the coefficient of zinc is: Zn + CuSO4 â†' ZnSO4 + Cu 1 2 3 4

Electrochemistry and Oxidation – Reduction - ppt video online download

Sel Volta | blueocean

PDF) Contact deposition of copper powders onto zinc in H2SO4-CuSO4 and H2SO4-CuSO4-ZnSO4 solutions and their morphology
Electrochemistry is a science that offers the solution to our energy needs

Metals - презентация онлайн

Net Ionic Equation for Zn + CuSO4 | Zinc + Copper (II) Sulfate - YouTube

SEL VOLTA ATAU SEL GALVANI - SMA Syarif Hidayatullah Grati

CALORIMETRY ENTHALPY CHANGES be able to define enthalpy

Zinc reacts with with CuSO4 according to equation Zn+CuSO4=ZnSO4 +Cu if excess of zinc is added of zinc is added - Chemistry - Some Basic Concepts of Chemistry - 13524689 | Meritnation.com

OneClass: M=#mol CuSO4/L solutionZn(s)+CuSO4(aq)–>ZnSO4(aq)+Cu(s)From the weight of copper produc…
Bmedic - :D | Facebook

Apakah reaksi-reaksi berikut termasuk redoks? Manakah yang bertindak sebagai oksidator dan - Brainly.co.id

Laporan termokimia
Solved 3. When the equation below is balanced, the | Chegg.com
Redoks dan Elektrokimia - Tanya MIPI

Classify the following reactions into different types: (i) A ? | Scholr™

fujn Pages 101 - 150 - Flip PDF Download | FlipHTML5

2012 Reaction Unit Types prediction names symbols coefficients

Applied Sciences | Free Full-Text | A Virtual Experiment for Learning the Principle of Daniell Cell Based on Augmented Reality

Reaksi Kimia : Pengertian Dan Ciri - Ciri Reaksi Kimia Beserta Contoh Reaksi Kimia | Teks.Co.Id

KIMIA: KESPONTANAN REAKSI REDOKS

SOLVED:Please help me to balance the following equations; 1. Magnesium and Oxygen Chemical equation for the reaction: Mg+O2 –> MgO 2. Zinc and Copper (II) Sulfate Chemical equation for the reaction Zn+CuSO4 –>

Reduction - Oxidation Chapters ppt download

Chemistry notes, Chemistry, Chemistry class


